The industry standard in biopreservation media
Success in the regenerative medicine, biobanking, and drug discovery industries depends on the shelf-life and viability of cells, tissues, and organs designated for research or clinical applications. We can help you be successful with best-in-class media products for your biologic source material, intermediates, and final products.
Media for each of the biopreservation modes
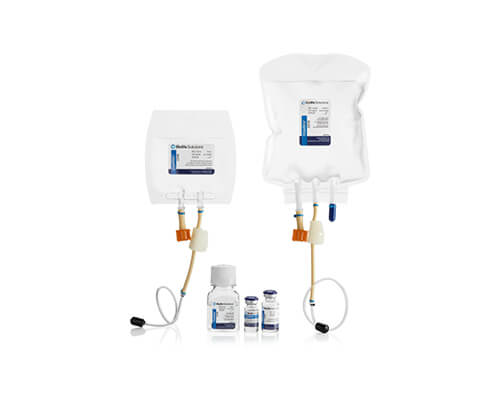
CryoStor®
Cryogenic storage
CryoStor proprietary freeze media products are intended for cryopreservation of biologics at -196 to -70°C. Across a broad spectrum of cell types, CryoStor formulations have proven more effective in reducing post-preservation apoptosis and necrosis than commercial and home-brew isotonic and extracellular formulations.
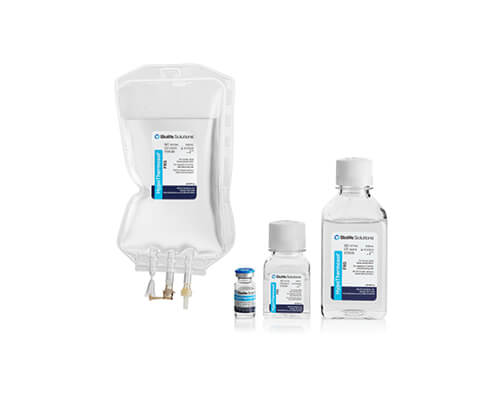
HypoThermosol® FRS
Non-frozen storage
HypoThermosol FRS (HTS-FRS) biopreservation media is a novel, engineered, optimized hypothermic storage and shipping media. Serum-free and protein-free, HypoThermosol is designed to provide maximum storage and shipping stability for cells and tissues at 2-8°C.
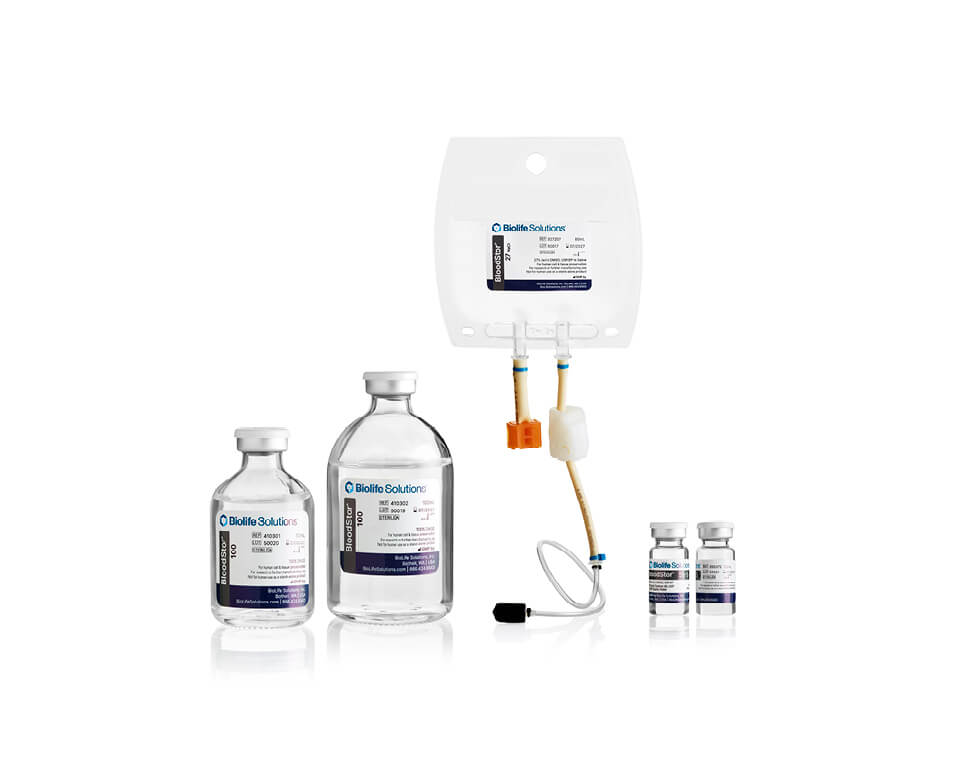
BloodStor®
Cryopreserve cells
BloodStor® is a pure media product that contains various levels of DMSO concentration from 27% to 100% DMSO, in Saline or Dextran. BloodStor has been sterilized, tested for endotoxins and manufactured under cGMPs with USP-grade components.

Cell Thawing Media
Cell and tissue thawing
BioLife’s Cell Thawing Media products originated from a Dextran shortage in the leukapheresis industry. Blood products need an added buffer for long-term storage.
Cryopreservation
Cryopreservation, a method that has been used for decades, employs extremely low temperatures to safeguard cells and tissues for subsequent utilization. This approach encompasses the cooling of cells to exceedingly low temperatures (-196˚C to 70˚C, thereby inducing a suspension of their cellular metabolism.
This suspension effectively preserves the cells indefinitely. The freezing process, if not carefully managed, can lead to an imbalance of solutes and structural damage within cells due to the formation of ice. By employing precise techniques and utilizing a freeze media comprised of appropriate cryoprotectants and additives, researchers can optimize the post-thaw viability of cells for subsequent cell culture.
Partner with us to optimize your bioprocess
Integrated solutions to automate and close your workflow
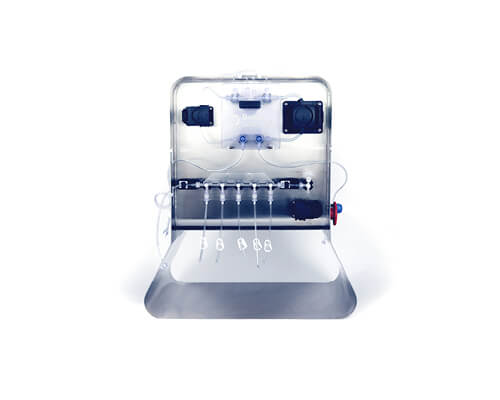
Signata CT-5®
Automated aliquoting for high-value cell products
The Signata CT-5 is a compact, benchtop aliquoting system that enables automated, aseptic filling into vials (including CellSeal® Cryogenic vials) without the need for a biosafety cabinet. With its intuitive touchscreen interface, pre-configured fill protocols, and compatibility with closed tubing sets, the Signata CT-5 minimizes operator intervention and enhances repeatability.
Key benefits:
- Reduces cleanroom footprint
- Enhances batch reproducibility
- Lowers operator time and error
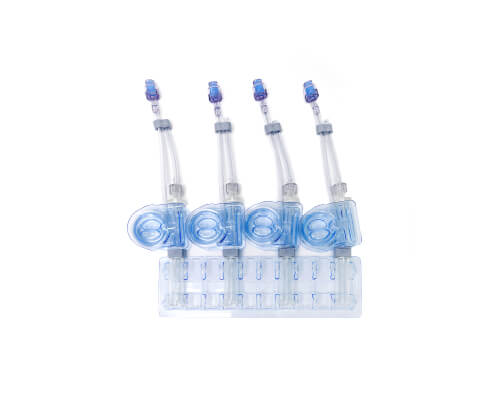
CellSeal® Connect
Seamless, closed-system transfer
CellSeal Connect advances the CellSeal platform with a weldable fill and retrieval tube. Designed with intermediate activation, transduction and expansion CGT processing in mind, these vials can be filled in a closed-system manner and sterile welded onto downstream processing systems.
Applications:
- Mid-process sampling
- Finished product fill/finish
- Cryopreserved cell transfer
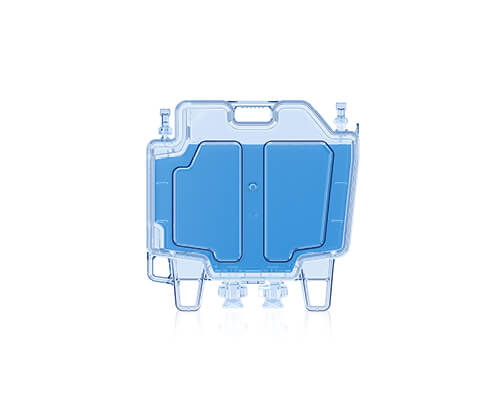
CellSeal® CryoCase
Redefining cryostorage standards
The CellSeal CryoCase is a rigid, cryopreservation container designed for cell and gene therapy (CGT) storage. It’s the first rigid container engineered for closed-system fill and retrieval of larger volumes of fluid (<75 mL) and it was developed to address limitations of traditional cryobags like the presence of particulates and the risk of leaks and fractures.
The CellSeal CryoCase has demonstrated:
- Improved inspectability
- Compatibility with closed-system processing
- Versatility with fill volumes
- Durability unseen before in primary container storage
Ask the
Scientists
Our team of experts have enabled biopreservation standardization and optimization for a diverse spectrum of customer applications and are available to answer any questions you may have.
Batch-specific documentation verifying product quality and compliance
BioLife Solutions products to be used in your clinical application
Specifications, certifications, and validation documents
An entire page of information dedicated to our CDMO partners