ThawSTAR® CFT Transporter
ThawSTAR® AT Transporter
ThawSTAR® CFT2 IOPQ Packet
ThawSTAR®
Confirmation Vials
ThawSTAR® CFT Transporter

Controlled intra-campus transport for cryopreserved vials
Comprised of an outer insulative foam lid and base, and an internal round CFT2 Core that accommodates up to five 1.8 or 2.0 mL (CFT2), or five 1.5 mL (CFT1.5) cryogenic vials.
Prior to using the ThawSTAR® CFT Transporter, a thin layer of dry ice is added to the bottom of the base. The round CFT2 Core is placed over the dry ice and allowed to equilibrate to dry-ice temperature (approximately 20 minutes). A frozen vial is then extracted from storage and quickly placed into the module. The lid is placed securely on top of the base and all samples are maintained at dry ice temperature until transported to the thawing location.
- Dimensions: 11.4 x 15.0 cm (4.5 x 6.0 in)
- Dry ice: Uses approximately 150 g of dry ice
- Holding temperature: Less than -70°C
- Holding time: Greater than 1 hour
- Vial capacity: Accommodates up to 5 vials (CFT2 Core)
ThawSTAR®
AT Transporter

Bridge the gap between storage and thawing
Comprised of an insulative foam base and lid, and an AT Core that accommodates six 2.0 mL, 6.0 mL or 10.0 mL AT-Closed Vials®.
ThawSTAR® AT Transporter is a portable cold storage solution for handling and transporting frozen cell therapy vials from long-term storage in vapor phase liquid nitrogen or in a -80°C freezer to downstream thawing along with the ThawSTAR AT Automated Thawing System.
- Compatibility: 2.0 mL, 6.0 mL, or 10.0 mL AT Closed Vials®
- Capacity: Accommodates one vial at a time for the thawing process
- Comprised of an insulative foam base and lid
- Dimensions: 20.0 x 16.0 x 16.0 cm (7.8 x 6.3 x 6.3 in)
- Dry ice used: 250g
- Holding temperature: Less than -70°C
- Holding time: Greater than 1 hour
- Dry ice used: Approximately 150g
ThawSTAR® CFT2
IOPQ Packet

IOPQ documentation for regulatory confidence
Qualification documentation and testing accessories based on GxP industry standards and the GAMP® 5 methodology.
Contents include:
- Comprehensive documentation makes therapeutic cell thawing easy to authenticate
- Testing accessories included with Qualification Packet
- Step-by-Step IOPQ protocol and report templates for thawing in a controlled environment
ThawSTAR®
Confirmation Vials
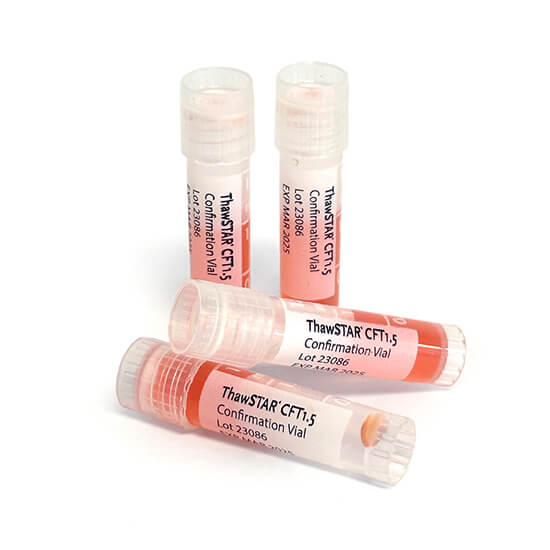
Precision-engineered reference tools
ThawSTAR Confirmation Vials are precision-engineered reference tools used to validate automated thawing performance. They simulate the thermal mass and geometry of standard cryovials, enabling consistent verification of thaw profiles and reproducibility across systems or operators.
- Mimics standard cryovial dimensions and contents for accurate calibration.
- Ensures repeatable thaw performance in ThawSTAR® CFT1.5 and CFT2 systems.
- Durable construction supports multiple validation cycles.
- Essential for compliance in regulated CGT manufacturing environments.