Biopreservation in CGT: Expertise that Safeguards Every Cell
For developers of cell and gene therapies (CGTs), biopreservation is far more than the simple storage of the product. It impacts various stages of the manufacturing process. The functional viability of cells post-thaw can determine the success of downstream workflows, clinical outcomes, and ultimately, commercial success. To learn more about the different biopreservation methods, check out our Mastering Biopreservation page.
Despite the essential role of biopreservation in cell and gene therapy manufacturing, there is a relatively small international pool of cryobiologists. This talent gap poses real risk to process reliability and scalability. The unique knowledge required to understand, control, and troubleshoot biopreservation-induced damage is rare, and few institutions train professionals in this hybrid discipline that spans biology, thermodynamics, chemistry, and engineering.
As demand for advanced therapies surges, many bioprocess teams are struggling to find experienced cryobiologists to lead their biopreservation efforts or mentor staff. In this environment, partnering with a provider that already maintains deep cryobiology expertise is not just advantageous, it is becoming increasingly indispensable.
At BioLife Solutions, we believe that preserving the promise of advanced therapies begins with biopreserving the cell. Our industry-leading biopreservation media and unmatched team of cryobiologists have set the global standard for biopreservation performance and reliability.
Whether you are troubleshooting low post-thaw viability, limited non-frozen stability, scaling from research to GMP production, or preparing for regulatory submission; BioLife Solutions provides the expertise, technology, and evidence-based guidance to support success.
The BioLife Bench of Cryobiology Expertise
Dr. Aby J. Mathew is a recognized thought leader in biopreservation for research, GMP, clinical, and commercial applications; with extensive expertise in cell and tissue biopreservation technologies.
He holds a B.S. in Microbiology and a PhD in Cell & Molecular Biology, and is a co-developer of the industry-leading HypoThermosol® FRS and CryoStor® biopreservation media platforms.
A driving force in Biopreservation Best Practices and the regenerative medicine field, Dr. Mathew has been instrumental in advancing the adoption of improved biopreservation methods and solutions for cell-based products. His contributions include multiple issued or pending patents, along with numerous peer-reviewed journal articles that continue to shape Biopreservation Best Practices in the industry.
Dr. Alireza Abazari is currently the Senior Director of Cell Processing at BioLife Solutions. In this role, his leadership is in expanding BioLife Solutions capacity to assist customers and clients with product adoption. A deep understanding of biopreservation principles as well as hands-on experience in CAR-T and iPSC process development from his past roles at Pluristyx Inc. and Lyell Immunopharma, enables Alireza in his current role to offer a unique perspective to BioLife customers and clients.
Dr. Abazari received his PhD in Chemical Engineering from the University of Alberta where he studied methods for cryopreservation of human tissues for banking. He further studied biopreservation as a Postdoctoral Research Fellow at Harvard Medical School. He has authored more than 30 peer-reviewed journal articles, industry white papers, and abstracts on the topic of cryopreservation and process development.
Dr. Jason Acker is a distinguished leader in biopreservation and the former President of the Society for Cryobiology, with extensive experience bridging scientific research and industry innovation. He has dedicated his career to advancing cryopreservation technologies, blood component processing, and regenerative medicine applications.
With a strong background in academic research, regulatory compliance, and technology development, Dr. Acker has contributed to improving the quality, safety, and efficacy of cellular therapies worldwide.
Dr. Acker holds an MBA in technology commercialization and a PhD in Medical Sciences from the University of Alberta, where he has led their world-class cryobiology research and training programs for more than 15 years. His work has led to significant advancements in biobanking, process optimization, and cold chain logistics, ensuring the integrity of biological products from lab to patient. Through his leadership, mentorship, and scientific contributions, Dr. Acker continues to shape the future of cell therapy and biomanufacturing and is the Co-Founder and Director of PanTHERA CryoSolutions, now part of BioLife Solutions.
Dr. Robert Ben is Co‑Founder and Chief Scientific Officer of PanTHERA CryoSolutions, now part of BioLife Solutions, and a leading expert in cryopreservation and medicinal chemistry.
He holds a PhD in Synthetic Organic Chemistry from the University of Ottawa and has held academic positions at Binghamton University (State University of New York) and the University of Ottawa, where he was named Full Professor in 2015.
Dr. Ben’s research bridges organic synthesis, carbohydrate and peptide chemistry, and cell biology, with a central focus on designing novel cryoprotectants that inhibit ice recrystallization — a critical advance for improving the viability of cells and tissues used in regenerative medicine. His work continues to shape the future of biopreservation by helping developers protect the integrity of cells throughout storage, transport, and delivery.
Along with our cryobiologists on staff, we also partner with Dr. Erik Woods and Dr. Dayong Gao as part of our Scientific Advisory Board.
Protecting Cells at the Source: Intracellular Formulations for Low Temperature Stress
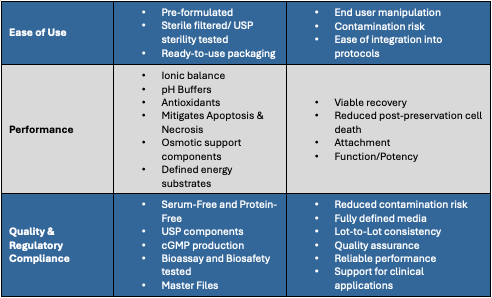
BioLife Solutions’ biopreservation media are pre-formulated, sterile filtered, and ready for immediate integration into cell-based workflows, limiting the need for reformulation or revalidation. This reliability becomes even more critical as operations scale across facilities or clinical phases, where consistency and control directly influence therapeutic outcomes.
Beyond convenience, our formulations are engineered to protect cells at the cellular and molecular level. They support ionic balance and pH stability, include antioxidant protection, and provide osmotic and metabolic support to minimize apoptosis and necrosis during hypothermic intervals, freezing and thawing. The result is superior post-biopreservation recovery and sustained cellular functionality.
Formulations are serum-free, protein-free, and manufactured per cGMP using USP-grade/Multi-compendia components, helping your biopreservation process align with clinical and regulatory guidance.
Why Controlling Ice Crystal Growth Is Critical to Cell Cryopreservation
Freezing is not the only threat to cell integrity. Uncontrolled ice crystal growth during thawing and transient warming events (TWEs) can be equally damaging. As Dr. Jason Acker presented at The Cell Summit ’25, brief exposures to non-cryogenic temperatures during transport or handling can drive ice recrystallization, producing larger, more destructive crystals that rupture membranes and impair potency.
Even small, repeated TWEs have been shown to cause measurable declines in viability and function. For instance, hematopoietic stem cells and red blood cells exposed to multiple warming cycles display increased hemolysis and reduced colony-forming capacity. In cell and gene therapy manufacturing, where every cell contributes to therapeutic efficacy, such losses can have critical downstream consequences.
Ice Recrystallization Inhibitors
Ice recrystallization inhibitors (IRIs) represent a new generation of cryoprotective innovation. Inspired by antifreeze proteins found in nature, these small-molecule compounds restrict ice crystal growth during both freezing and rewarming, maintaining a stable microstructure that preserves membrane integrity. By mitigating the physical stresses that compromise viability, IRIs enhance post-thaw cell recovery and function, even when real-world conditions introduce temperature variability. As the CGT field advances, IRIs may redefine what it means for a sample to be not merely frozen but truly preserved.
Freeze Smarter: Why Biopreservation Strategy Matters More Than Ever
Biopreservation is not a procedural afterthought; it is a critical determinant of cell quality and manufacturing success. Home-brew reagents or ad-hoc freezing approaches may not ensure the precision, reproducibility, and regulatory compliance required in today’s cell therapy programs. Partnering with experts who understand cryobiology at its core enables organizations to safeguard cell functional integrity, shorten development timelines, and reduce risk from early process design through application of the cell products (research, clinical, commercial).
At BioLife Solutions, our mission is to support Biopreservation Best Practices by equipping teams with scientifically validated media, deep cryobiology expertise, and hands-on process support that helps every preserved cell retain its therapeutic promise.
Looking to improve post-thaw viability or reduce risk in your biopreservation workflow?
Start with the experts at BioLife Solutions. www.biolifesolutions.com/askthescientists
Want to see what experts across the industry are doing?
Download our Cell Summit Proceedings eBook for insights and real-world practices from today’s CGT leaders.
References
- Mathew, A. J. (2025, August). Navigating biopreservation regulatory considerations… but why limit our discussion to that… Presented at The Cell Summit ’25, Indianapolis, IN.
- Acker, J. P. (2025, August). Frozen doesn’t mean stable: Controlling ice recrystallization is critical to quality. Presented at The Cell Summit ’25, Indianapolis, IN.



