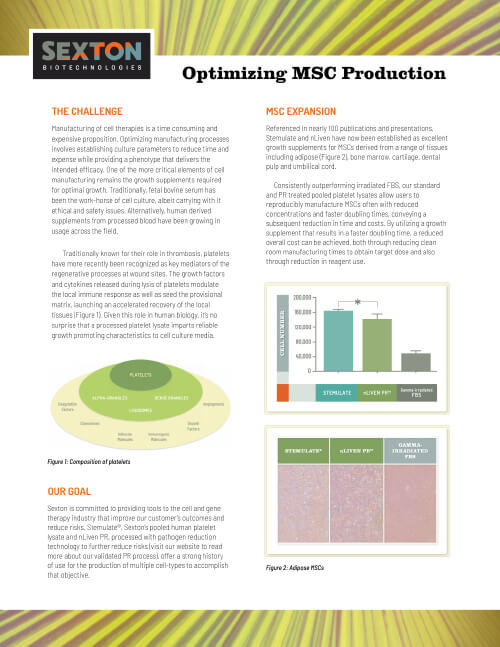
Case Studies & White Papers
BioLife Solutions’ and other experts bring you insight and analysis on today’s industry best practices in biotechnology, biopreservation, and cold chain management.
Biopreservation Today® Archive
This is our archive for the FREE magazine highlighting key insights and industry trends in the world of biopreservation and cold chain shipping we used to publish between 2009 and 2019. This initiative has since been replaced by a monthly blog cadence, but the archives may still be useful for reference.
2019: Winter
Volume 9, Issue 1
Feature article
Biolife customers continue path towards commercialization:
- BioLife Solutions Scientists Partner with the Parenteral Drug Association to Advance Biopreservation Best Practices
- Extending Shelf Life and Minimizing Logistics Challenges in Starting Materials for Clinical and Commercial Development
- Ask the Scientists
- SAVSU Technologies News
- Explore BioLife’s Evidence Library.
2018: Spring
Volume 8, Issue 1
Feature article
Dead cells don’t cure cancer: why effective biopreservation is critical for commercialization of cell therapies:
- Cold Chain Corner
- Ask the Scientists
- BioLife vs. Homebrew Cryopreservation Media
- Transitioning to Cryopreservation Media Increases Post-Thaw Recovery
2017: Spring
Volume 7, Issue 2
Feature article
Biolife Solutions and ISCT – celebrating 25 years of advancing cell therapies together:
- Ask the Scientists
- Learning from Regenerative Medicine Customers Poised for Commercialization
- Biotechnology Industry Growth Gives Rise to a New Generation of Industry Resources
2017: Winter
Volume 7, Issue 1
Feature article
Cold chain 2.0: the intelligent transportation workflow for time and temperature sensitive biologics:
- biologistex Joint Venture Restructuring
- evo Surpasses Rigorous Testing Criteria
- Growing Body of Biopreservation Performance Evidence
2016: Spring
Volume 6, Issue 1
Feature article
Establishing best practice qualification metrics for smart shipping containers:
- Veterinary Regenerative Medicine
- MNX® Global Logistics Partnership
2015: Fall
Volume 5, Issue 2
Feature article
Chain Boosts Support of Advanced Therapy Products:
- New Product Updates
- Are Cryopreserved Platelets Just Around the Corner?
- Meet the BioLife Solutions Scientific Team
2015: Winter
Volume 5, Issue 1
Feature article
Web-Connected Biologistics – Cold Chain Lessons Learned From the Pharmaceutical Industry:
- Smart Shippers Come of Age
- Bags and Syringes
- Biopreservation of Cellular Immunotherapies
2013: Fall
Volume 4, Issue 4
Feature article
BioLife Products in Regenerative Medicine Applications:
- New Products Preview-Pre-Filled Syringes and Bulk Media in Bags
- Opportunities and Challenges in 3D Bio-Printing
- SAVSU® Precision Thermal Packaging Overview
2013: Spring
Volume 4, Issue 3
Feature article
What’s Up Down Under:
- Alliance for Regenerative Medicine
2013: Winter
Volume 4, Issue 2
Feature article
Biolife Products In Regen Med Clinical Trials:
- Biolife Solutions Contract Media Manufacturing
- Biolife Solutions Completes Validation Of 2nd Cgmp Clean Room Manufacturing Suite
- Global Frozen Shipping Solution For Life Sciences
2012: Summer
Volume 4, Issue 1
Feature article
Continuous Improvement And Best Practices in Biopresevation Of Cell And Tissue Based Products And Therapies:
- Validation Of In-house Quality Assurance Assays
- Biolife Solutions Commences Build-out Of Second Gmp Production Suite And Corporate Office Expansion
2011: Spring
Volume 3, Issue 1
Feature article
HypoThermosol® and CryoStor® Excipient Reagents in Regenerative Medicine:
- Getting The Most Out of Supplier Quality Audits
- Multistem™ – Extending Final Dose Stability with Hypothermosol®
- Evaluation of Vavelta® In Burns, Wound Scarring, and A Genetic Skin Disorder
- Adoption of Cryostor® In Manufacturing of A Dendritic Cell Vaccine Platform
- Optimizing Manufacturing of Hospital-Based Cellular Therapies to Support Clinical Trials
2011: Winter
Volume 2, Issue 4
Feature article
Bringing a Regenerative Medicine Product to Market – Contract Manufacturing Organizations Improve Probability of Success:
- Cell Therapies Pty, Ltd Profile
- Lonza Bioservices Profile
- PharmaCell BV Profile
- Progenitor Cell Therapy, LLC Profile
2010: Fall
Volume 2, Issue 3
Feature article
Development and Commercialization of New Regenerative Medicine Products; the Crucial Role of Consultants:
- New Comparative Preservation Data
- Advanced Gene and Cell Therapy Profile
- DCi-BIOTECH Profile
- Biologics Consulting Group, Inc. Profile
- Compliance Consulting Profile
- Cell Therapy Group Profile
- HypoThermosol® Adoption in Hair Transplantation
2010: Spring
Volume 2, Issue 2
Feature article
Biopreservation Economics in Regenerative Medicine:
- Origen Biomedical Profile
- Ancillary & Excipient Reagents
- American Fluoroseal Corp. Profile
- Saftey Study of Intravenous Injection of Biopreservation Solutions
2010: Winter
Volume 2, Issue 1
Feature article
“Home-Brew” Biopreservation Solutions:
- Cryopreservation Storage Study using the CellSeal™ Cryogenic Container System and Serum-Free CryoStor™ Preservation Solution
- BioLife Solutions is ISO 13485 Certified
- BioLife Solutions Announces Custom cGMP Manufacturing and License Agreement with Centocor
2009: Fall
Volume 1, Issue 4
Feature article
Case Study: Modified Cryopreservation of Umbilical Cord Blood Stem Cells at Cryobanks International by Aby J. Mathew, PhD, Director of Strategic Relations and Senior Scientist, BioLife Solutions and Donald L. Hudspeth, BSCLS, MT(ASCP), General & International Projects Manager, Cryobanks International, Inc.
- Educational Opportunities at the 2009 AABB Annual Meeting & TXPO
- Improved Method for Collection and Stability of Umbilical Cord Blood Prior to Processing
2009: Summer
Volume 1, Issue 3
Feature article
The Evidence-Based Biobanking Movement by Lisa Miranda, President, Biobusiness Consulting, Inc.
- Researchers Report Significant Advantages of CryoStor™
- BioLife Launches GMP Production Facility
- Evolution of Best Practices in Biopreservation: Part 3
- BioLife Animation Draws Attention
- Improved Recovery and Function of Peripheral Blood Stem/Progenitor Cells
2009: Spring
Volume 1, Issue 2
Feature article
Biopreservation Stability Considerations for Cell Therapy Development and Commercialization by Scott Burger, MD, Principal, Advanced Cell & Gene Therapy, LLC
- How Safe are Your Reagents?
Biopreservation Economics
Evolution of Best Practices in Biopreservation: Part 2
2009: Winter
Volume 1, Issue 1
Feature article
Unlocking the Value of a Scientific Advisory Board by Mike Rice, Chairman & CEO, BioLife Solutions, Inc.
- What is your Viability Assay Really Saying?
- Optimizing Biopreservation Yield
- Evolution of Best Practices in Biopreservation: Part 1 Profile
CDMO Resources
BioLife Solutions partners contract development and manufacturing organizations (CDMOs) to provide robust support for advanced therapy production. Whether you’re streamlining your internal processes or supporting client submissions, our resources are designed to meet your unique challenges.
Key materials
- GMP product documentation
- Regulatory support tools for IND/BLA readiness
- Batch records and quality assurance documents
- Application notes on automated fill/finish and cryopreservation
- Case studies on scalability and closed-system adoption