Protecting Cell Therapy Quality by Preventing Transient Warming Events
As cell and gene therapies continue to gain traction, the industry’s focus on automation, digital transformation, and advanced manufacturing platforms is stronger than ever. But in the midst of these innovations, one often overlooked challenge can quietly compromise therapy outcomes: Transient Warming Events (TWEs).
At The Cell Summit ‘25, speakers across the cell and gene therapy (CGT) ecosystem repeatedly highlighted TWEs as a critical risk factor. Whether caused by delays in shipping, poor storage handling, or inconsistent transport protocols, these subtle temperature fluctuations can have a significant impact on cell viability, potency, and consistency on cell products.
What Is a TWE?
A Transient Warming Event occurs when a cryopreserved sample is exposed to warmer-than-intended temperatures for a short period of time. This temperature excursion might seem insignificant at first, but even brief exposure to higher temperatures can trigger a cascade of biological stress inside the cells.
Common causes of TWEs include:
- Transfers between storage systems or freezers
- Delays or mishandling during shipping and receiving
- Repeated opening of cryogenic freezers for inventory access
- Improper thawing procedures or equipment downtime
These events often go undetected without continuous temperature monitoring or robust cold chain management protocols.

Why Are TWEs So Dangerous for Cell Therapy?
Even in frozen or ultra-cold conditions, cells are not biologically inert. Shipment excursions and warming events can activate harmful processes inside the sample, leading to reduced functionality or therapeutic failure. This graphic from Cytiva does an excellent job outlining the effects temperature has on a cell.
Here is how a TWE may impact your sample:
- Ice Recrystallization: Ice crystals can grow during warming, damaging cell organelles membranes.
- Osmotic Stress: Water movement into or out of cells becomes unbalanced, leading to structural instability.
- Cryoprotectant Toxicity: DMSO, HES, and other cryoprotectants become more toxic as temperatures rise.
- Delayed Onset Cell Death (DOCD): Even if immediate post-thaw viability appears high, cells subject to repeated TWE may undergo apoptosis hours or days later due to cumulative stress.
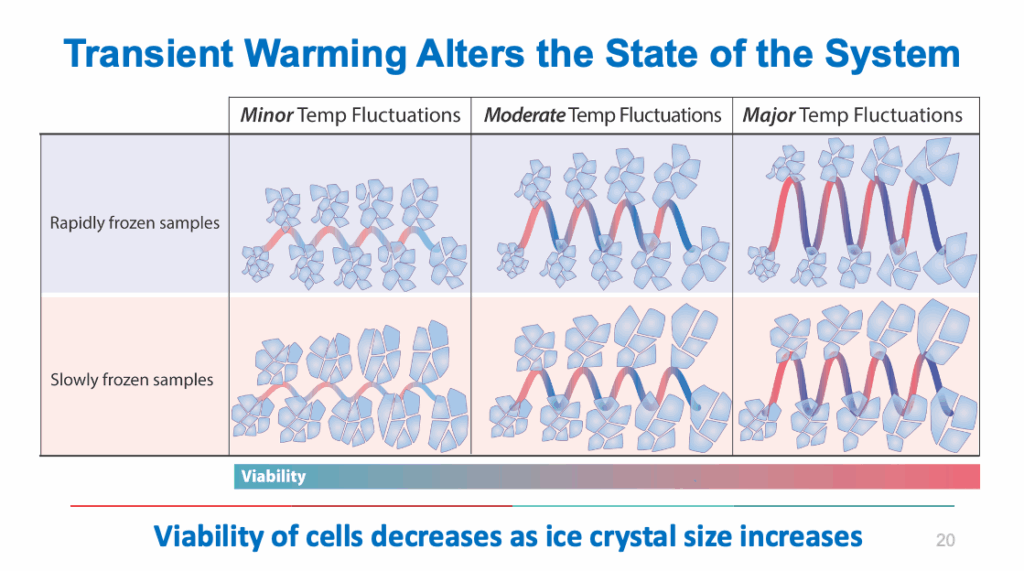
In cell therapy, where every cell counts and every dose is critical, a TWE can mean the difference between a successful treatment and an unusable product.

Throughout The Cell Summit ‘25 event, discussions on TWEs emerged as a consistent concern:
- Bruce Thompson, PhD (Kincell Bio) emphasized that TWEs are a primary source of post-thaw variability, often undetectable without delayed functional testing. (Thompson, 2025)
- Ashley Krull, PhD (The Ohio State University) described how academic labs often lack the infrastructure to monitor and prevent warming events in shared storage environments. (Krull, 2025)
- Matt Branch, PhD (King’s College London) shared his team’s challenges with vial options and cryopreserving delicate cone photoreceptor cells, underscoring the need for consistency across freezing and thawing. (Branch, 2025)
- Alex Sargent, PhD (Charles River Laboratories) presented data demonstrating how variations in thaw protocols and container type can impact recovery and function—even when using high-end equipment. (Sargent, 2025)
- Erik Woods, PhD (Ossium Health) called for improved storage protocols and stability testing, presenting evidence that thermocycling from -135°C to -60°C led to significant losses in cell viability and function. (Woods, 2025)
- Jason Acker, PhD (University of Alberta) demonstrated how even short warming episodes lead to ice recrystallization and cell damage, highlighting the protective role of IRIs to mitigate these effects. (Acker, 2025)
- Olga Bukatova, PhD (Azenta Life Sciences) shared best practices for controlled thawing in GMP and clinical environments, emphasizing the importance of rapid, uniform warming to avoid recrystallization and maintain consistency. (Bukatova, 2025)
Across the board, speakers agreed that TWEs are preventable yet remain one of the most damaging and under appreciated risks in cell therapy workflows.
How to Prevent TWEs in Cryopreserved Cell Products
To protect your CGT product from shipment and temperature excursions, follow these best practices:
Use real-time data loggers and sensors in freezers, storage units, dry shippers, and transport systems to detect any warming events immediately.
As shared by Jason Acker (University of Alberta) at The Cell Summit ’25, IRIs can dramatically reduce the damage caused by transient warming. These nature-inspired molecules inhibit the growth of ice crystals that would otherwise expand and rupture cell membranes during brief warming episodes. Acker presented data showing that IRIs help preserve post-thaw potency and cell quality even after multiple warming cycles.
Develop and enforce standard operating procedures for all cryogenic handling activities, including shipping, receiving, and internal transfers.
Using cryogenic containers with high thermal mass, like CellSeal® CryoCase, may extend safe handling windows and minimize heat transfer.
Ensure that all staff, from lab technicians to shipping couriers, understand the risks of temperature excursions and how to avoid them.
Track every movement, monitor every shipment, and include TWE assessment as part of your lot release criteria.
TWEs Are Invisible, But Their Impact Is Not
Transient Warming Events may not show up immediately post-thaw in standard membrane integrity assays. But their impact on cell quality, phenotype, and potency is significant. If you do not control TWEs, you introduce avoidable cell damage into your therapy pipeline—and in cell therapy, variability can be catastrophic.
The good news is that TWEs are preventable. With the right tools, processes, and vigilance, you can build a biopreservation program that protects every cell and delivers consistent therapeutic performance. We will be issuing a blog series on the topic of Ice Recrystallization Inhibitors (IRIs) and will be sharing our efforts to reduce TWEs.
Final Word: Preserving the Promise Starts with Preserving Temperature Integrity
Cell and gene therapies are transforming lives. But to truly deliver on their promise, we must preserve not just the cells, but the conditions that keep them viable and functional. That includes recognizing and eliminating and providing protection from TWEs, managing shipment excursions, and ensuring the cold chain is as robust as the science itself.
Protect your cells. Avoid TWEs. Preserve the promise.
References
- Acker, J.P. (2025, August). Frozen doesn’t mean stable: Controlling ice recrystallization is critical to quality. Presented at The Cell Summit ’25, Indianapolis, IN.
- Branch, M. (2025, August). Standardizing cryopreservation for fragile photoreceptor cells. Presented at The Cell Summit ’25, Indianapolis, IN.
- Bukatova, O., & Musall, K. (2025, August). Best practices in thawing to maximize cell recovery and function. Presented at The Cell Summit ’25, Indianapolis, IN.
- Krull, A. (2025, August). Navigating infrastructure gaps in academic cryostorage. Presented at The Cell Summit ’25, Indianapolis, IN.
- Sargent, A. (2025, August). Cryocontainer variables in thaw recovery: Implications for reproducibility. Presented at The Cell Summit ’25, Indianapolis, IN.
- Thompson, B. (2025, August). Sources of post-thaw variability: A delayed functional assay perspective. Presented at The Cell Summit ’25, Indianapolis, IN.
- Woods, E.J. (2025, August). Process optimization considerations: Storage and controls in cryopreserved cell therapies. Presented at The Cell Summit ’25, Indianapolis, IN.