Cryopreservation has come a long way...
Over the past 50 years, advances in biopreservation techniques have enabled industry-wide progress in a range of fields including cell biology research, drug discovery, biobanking, assisted reproduction, and even plant and animal conservation, with the fields of cellular therapy and regenerative medicine seeing the greatest expansion recently. As these industries continue to expand their collections of cryopreserved samples, the need for minimizing variability in sample handling through automation and standardization has become essential.
This means that researchers are not only focusing on improving R&D and freezing biologic materials, but they are also giving more attention to another important step in the biopreservation process: sample thawing. The thawing step is critical.3 As scientists have found, thawing protocols and equipment can add substantial variability to the biopreservation process and negatively impact cell viability, if done haphazardly.
For high-stakes areas like cellular therapy and regenerative medicine, standardizing the thaw process to drive repeatable and reliable protocols will be critical to ensure these therapies work as intended when administered to patients.
Why is Thawing so Important?
To understand thawing, it helps to start with the basics of freezing. In its most basic form, effective freezing requires controlled-rate cooling of cells, generally mixed with a cryoprotectant, to allow:
Minimization of intracellular ice crystal formation during the liquid-to-solid phase transition by effective cell dehydration. Control of osmotic gradients across the cell membrane as extracellular solute concentrations increase.
Limiting extracellular ice crystal expansion.
The microscopic processes occurring during thawing are almost mirror images in the opposite direction of those that occur during freezing: warming of the sample from cryogenic temperatures toward the solid-to-liquid phase transition, melting of extracellular ice crystals to form liquid water, rehydration of the cells, and reformation of an extracellular salt and protein solution.4
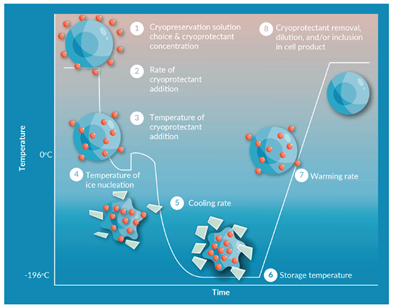
During a thaw, it is critical to minimize both osmotic shock to the rehydrating cells and overall ice recrystallization in the thawing mixture.1 Ice recrystallization during thawing is a commonly observed phenomenon where small ice crystals, generated during the freezing process, can reform into larger crystals at sub-freezing temperatures and then serve as sites where liquid water starts to form when the temperature rises.2 Ideally, the thawing rate and temperature should also be optimized for cell size and volume, cell type, and choice of cryoprotectant, but methods for automated cryovial thawing have been developed to ensure standardized sterile and reproducible cell recovery after cryopreservation.3
Current Thawing Methods
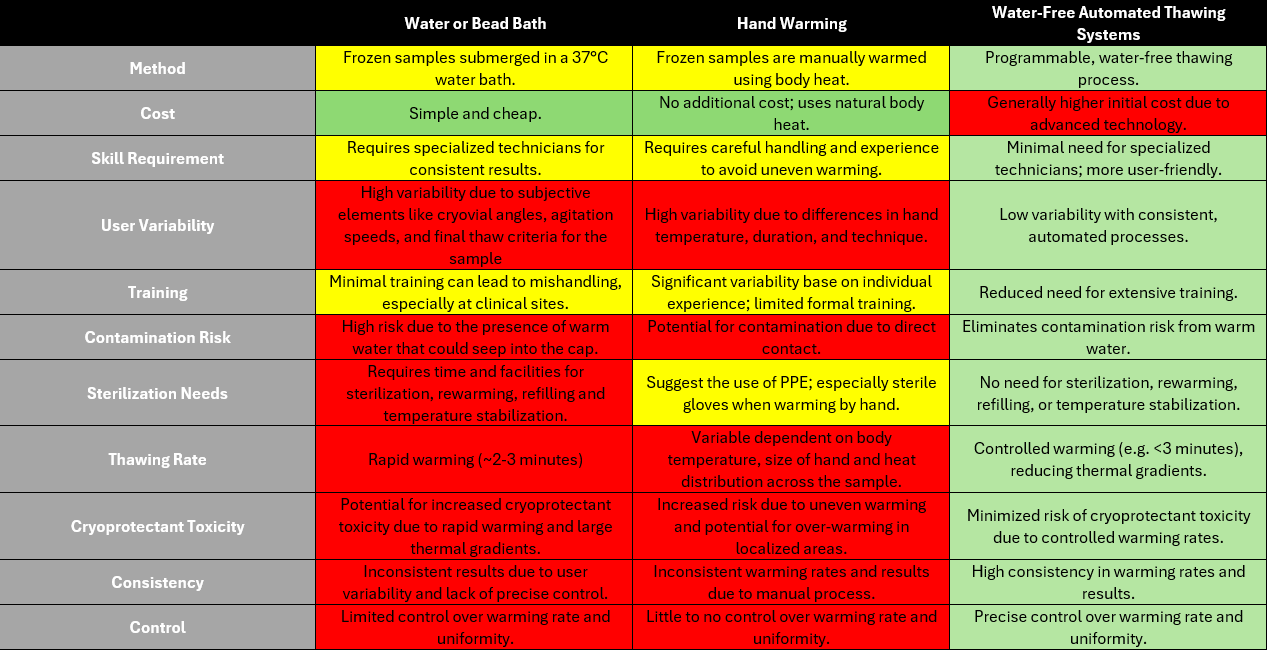
At present, there are three different methods for thawing a research sample: 1.) Warm water or bead bath, 2.) Hand warming a vial, or 3.) Water-free automated thawing systems.
Water/bead bath
For the water/bead bath technique, a frozen sample is typically held in a warmed bath of either water or glass beads that has been warmed to 37°C. After a few minutes, a visual inspection would confirm that all ice has melted. NOTE: Do not completely submerge a vial into a water bath. Water is not supposed to touch the cap to avoid any potential contamination risks. For more information on the water/bead bath technique, watch this video from Charles River Laboratories.
Hand warming
The hand warming method is just as it sounds – the user will take the sample vial from the freezer, let it sit for 3-5 minutes or until initial frost is melted, and hold it upright in their hand without a glove until all ice has melted; relying on body heat to normalize the warming rate.
Automated thawing systems
Water-free, automated vial thawing units, like the ThawSTAR® Automated Thawing Systems, are compact and use solid-state heating blocks with a pliant conductive material interface to maximize contact to vials being thawed. The ThawSTAR technology monitors the vial temperature during initial heating and detects the point at which the contents initiate phase change from solid to liquid. The result is a reproducible and standardized thaw for vials.
Each of these methods have pros and cons. The table below compares each method based on our in-house laboratory experience.
ThawSTAR CSV is NOW AVAILABLE
Recently, for our CellSeal® Cryogenic Vial and CellSeal® Connect™ users, we released a ThawSTAR vial thawing system compatible with those vials specifically. It has the same warming technology and controls as the other ThawSTAR models, but now applies those algorithms to CellSeal products.
ThawSTAR CSV Product Specifications
- Compatible with 2.0 mL and 5.0 mL CellSeal vials
- Thawing time: <3 minutes
- Dimensions: 10.9 cm x 14.5 cm (4.3 in x 5.7 in) D x H
- Power Rating: 12V DC, 3.0 A
- CE mark (IEC61326 and IEC61010), RoHS, WEEE
ThawSTAR Automated Thawing Systems can be part of a qualifiable operation, scalable across multiple point of use facilities. As precision medicine continues to grow, review your current thawing process. If your process has high user variability, low consistency and control, and contamination risks, it may be time to consider alternative automated methods. High dollar patient samples and patients deserve the best possible outcome during treatment.
For more information on automated cell thawing, reach out to our team of experts.
References
- BioLife Solutions (2019) ThawSTAR® Automated Cell Thawing System: Standardizing thawing using breakthrough solid-state technology.
- Cao, E. et al. (2003) Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol Bioeng. 82(6):684-90.
- Betsou, F., Gaignaux, A., Ammerlaan, W. et al. Biospecimen Science of Blood for Peripheral Blood Mononuclear Cell (PBMC) Functional Applications. Curr Pathobiol Rep 7, 17–27 (2019). https://doi.org/10.1007/s40139-019-00192-8
- Creer MH, Lemas MV, Mathew AJ (2015a) Practical Handbook of Cellular therapy cryopreservation. AABB Press, Bethesda, MD