The cell and gene therapy (CGT) industry is experiencing tremendous translational and clinical success, which has enabled commercial realization. This success has also paved the way for a wealth of new companies and clinical trials targeting further advancement in the treatment of life-threatening diseases. However, despite its success, the industry is addressing ongoing challenges.
A key challenge to the increase in clinical product development is the availability and management of incoming starting material and subsequent manufacturing capacity. However, the limited availability and overall stability of starting materials place a premium on supply chain and logistics to enable greater access, and ensure the highest quality can be achieved.
Challenges with Current Starting Material Management Practices
A number of factors influence the starting material quality, and that quality subsequently impacts each of the downstream steps. Once the material is collected (whether autologous or from a donor), the product needs to be delivered to a processing facility, which may be the sponsor’s production site or a contracted manufacturer (CMO/CDMO) for process development or further manufacturing.
Much like CGT final products, starting materials have living components that must remain in a stable state, free from the ravages of metabolic processes or chemical reactions to be effective. This may make starting material logistics and shelf-life challenging. Under current practice, starting material (development and clinical) is collected from the donor or patient when they are available, a time which is not always known. Logistics to transport the material need to be arranged, and processing facilities, such as the sponsor’s manufacturing site or contracted CMO, need to have personnel and space available to accept the material. Because these products are typically used in a non-frozen state, they need to be transported and processed quickly to minimize loss of cell viability and function. This current model and practice can most certainly impact quality, but it also places a significant strain on manufacturing site flexibility, personnel, and capacity. Furthermore, the overall quality of the cell therapy starting material is compromised – especially if delays occur.
Solutions for Extending the Shelf-Life of Starting Materials
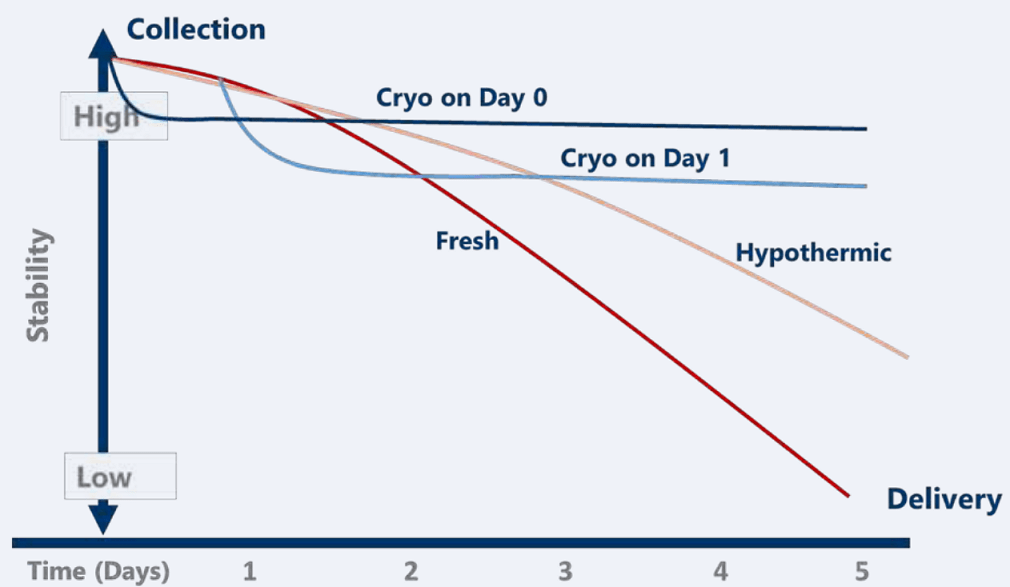
The CGT industry has placed a strong emphasis on the supply chain and logistics of the final therapeutic product. As many of these clinical products are manufactured at a remote facility and then transported long distances to a recipient awaiting treatment, cold chain and cryopreservation have become common practice to provide extended stability and preserve the critical quality attributes of the product. Fresh products ultimately have a limited shelf-life as biological material viability declines over time. For starting materials, receiving and processing “fresh” non-frozen materials 24 to even 48 hours, post-collection, is currently common practice. Cell loss is accepted, but delays in obtaining and receiving the product are also common, and the impact to the stability can be significant as depicted in the illustration (Figure 1).
Figure 1: Stability Impact on Starting Material Quality
Extending the stability of the starting material with biopreservation media offers alternatives to traditional industry practice for starting materials:
- Hypothermic medium: Including a hypothermic storage medium such as HypoThermosol® FRS (HTS-FRS) at the point of collection may extend the stability window by days and much longer if continuously stored at hypothermic temperatures (+2 to +8°C). Hypothermic preservation is employed to reduce metabolism and the need for oxygen and nutrients, extending the stability (shelf-life) long enough to get the product to the patient. With its optimized formulation, HypoThermosol® FRS mitigates temperature-induced molecular cell stress responses that occur during chilling and rewarming of cells and tissues, improving post-thaw viability for a wide range of cell and tissue starting materials.
- Cryopreservation medium: While cryopreservation offers indefinite stability (figure 1), it is important to understand that optimal cryopreservation of cells is not simply a matter of lowering the temperature below freezing. As freezing continues into the cryogenic range, more water solidifies out of the solution in the form of ice. Growth of intracellular ice can physically rupture membranes as cells in the unfrozen fraction are also exposed to harm from increasing salinity, with actual onset of cell damage and cell death occurring during post-preservation and rewarming.
The use of cryopreservation media, such as CryoStor®, is proven effective at mitigating damage from the formation of ice across a broad spectrum of cell types, reducing post-preservation apoptosis and necrosis beyond use of “home-brew” freezing media and DMSO cryoprotectant. Combining the use of cryopreservation media with slow, controlled freezing rates allows cells to respond osmotically and reduce the potential for intracellular ice formation, a major factor in maintaining cryogenic post-thaw viable cell recovery.
Benefits of Extending the Shelf-Life of Starting Materials
Optimizing the end-to-end process by using Biopreservation Best Practices that integrate the latest tools and technologies reduces the need for rapid processing and transportation while helping to maintain quality of starting materials. This not only increases the probabilities for clinical success, but also leads to improvements in downstream manufacturing efficiency and product consistency.
As more products are developed, and clinical trials increase, the current model for managing the starting material may continue to evolve in an effort to support growth of the cell and gene therapy industry. Without quality starting materials, the development of a critical, life-saving cell and gene therapy may be severely compromised before it even begins. Evaluating biopreservation methods to offer improved stability may potentially transform current practice while enabling improved product quality and availability.
Reference
Clarke, D. M. (2019). Extending Shelf Life and Minimizing Logistics Challenges in Starting Materials for Clinical and Commercial Development. Biopreservation Today. Retrieved 2023, from https://www.biolifesolutions.com/wp-content/uploads/2022/11/BPT_Winter_2019_SINGLES_REL.pdf.