BioLife Solutions acquired Sexton Biotechnologies to better serve the needs of cell and gene therapy (CGT) developers and manufacturers. Sexton complements BioLife’s existing portfolio by offering media to improve cell performance and cell expansion, automated and flexible fluid transfer manufacturing equipment, and modern CGT storage containers for closed-system processing. Sexton tools can advance your downstream processes, and CGT experts like Sean Werner, the BioLife Solutions Chief Technology Officer named in the interview summary below, are available to help.
Assessing your CGT Workflow
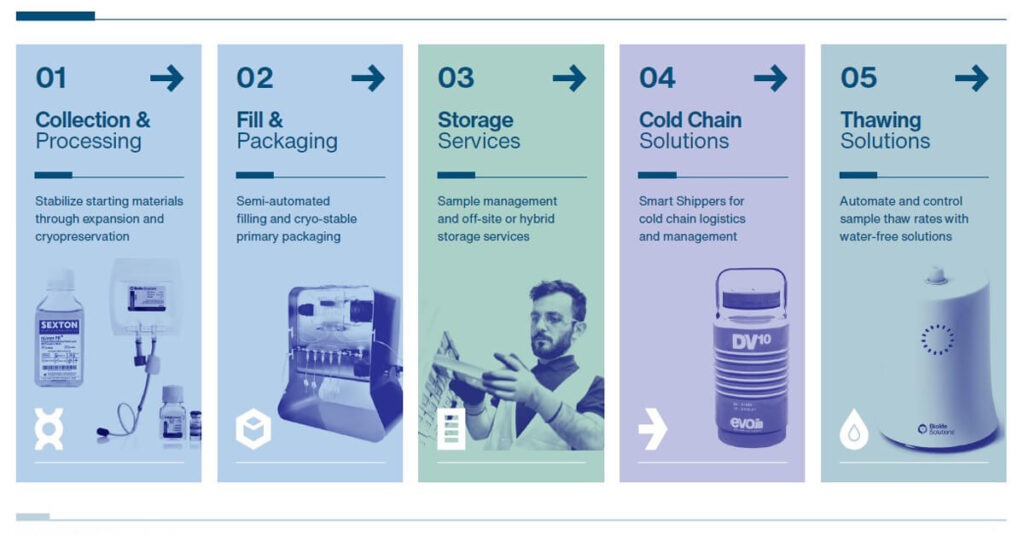
Creating a therapy that is specific to one patient is different than creating a therapy that will treat hundreds of patients repeatedly. There are ways to improve the R&D and manufacturing of both types of therapies through careful review of each process. BioLife Solutions can help address complexities in each of these steps:
Collection & Processing: Stabilize your starting materials through cell expansion and cryopreservation media to strengthen cell performance and viability.
Fill & Packaging: Look for semi-automated fluid filling systems and unique cryogenic or ultracold-stable storage containers for flexible intermediate processing.
Storage Services: Contemplate cGMP sample management in offsite facilities or hybrid options to reduce in-house cold storage equipment maintenance and upgrade costs.
Cold Chain Solutions: Transport your samples in wireless “Smart Shippers” that track shipments in real-time and record data for traceability.
Thawing Solutions: Automate and control your sample thaw rates with reproducible, water bath-free systems to maintain consistency of samples.
Cell Processing and Fill & Handling Tools

 BioLife Solutions focuses on providing you with tools that enhance cell performance, reduce risk, and increase the probability of positive clinical outcomes. Our goals are to improve cell processing methods, moving from lab practices to industry standards and to accelerate cellular performance with new reagents and techniques.
BioLife Solutions focuses on providing you with tools that enhance cell performance, reduce risk, and increase the probability of positive clinical outcomes. Our goals are to improve cell processing methods, moving from lab practices to industry standards and to accelerate cellular performance with new reagents and techniques.
- Pathogen reduced human platelet lysates (hPL) provide you with a heparin-free media that reduces risks and boosts downstream performance compared to FBS and other chemically defined options.
- The Signata CT-5™ platform brings multiple manual techniques under a higher level of control with flexible and semi-automated filling of cryobags and cryovials.
- The AF-500 automated fill and seal system provides a path to scale up and standardize through increased accuracy, speed, and productivity.
- CellSeal® Cryogenic Vials, CellSeal® Connect Closed-System (ULT) Vials and filling accessories offer multi-purpose storage of autologous and allogeneic products.
Interview with Sean Werner: Summary
(To read the full interview, click the link at the bottom of this page.)
In the first part, we discuss the new adventure of Sexton joining BioLife Solutions and the most pressing CGT problems to solve. With our combined expertise, we focus on CGT risk mitigation, biopreservation best practices and cell viability post-thaw.
Next, we review BioLife tools that can fill gaps in downstream processing and how we differ from other CGT suppliers. Last, we encourage suppliers to work together to elevate best practices and to solve complex issues to move research forward.
BioLife Solutions is committed to providing bioproduction tools and services to help researchers build the life-changing therapies of tomorrow. To realize the full potential of these therapies, new downstream solutions must be developed, evaluated, and brought to the life science industry to ensure robust, dependable, and cost-effective therapeutics can be administered to patients across the globe.