Optimizing Biopreservation Strategies for Apheresis-Derived Cells in Immunotherapeutic Applications
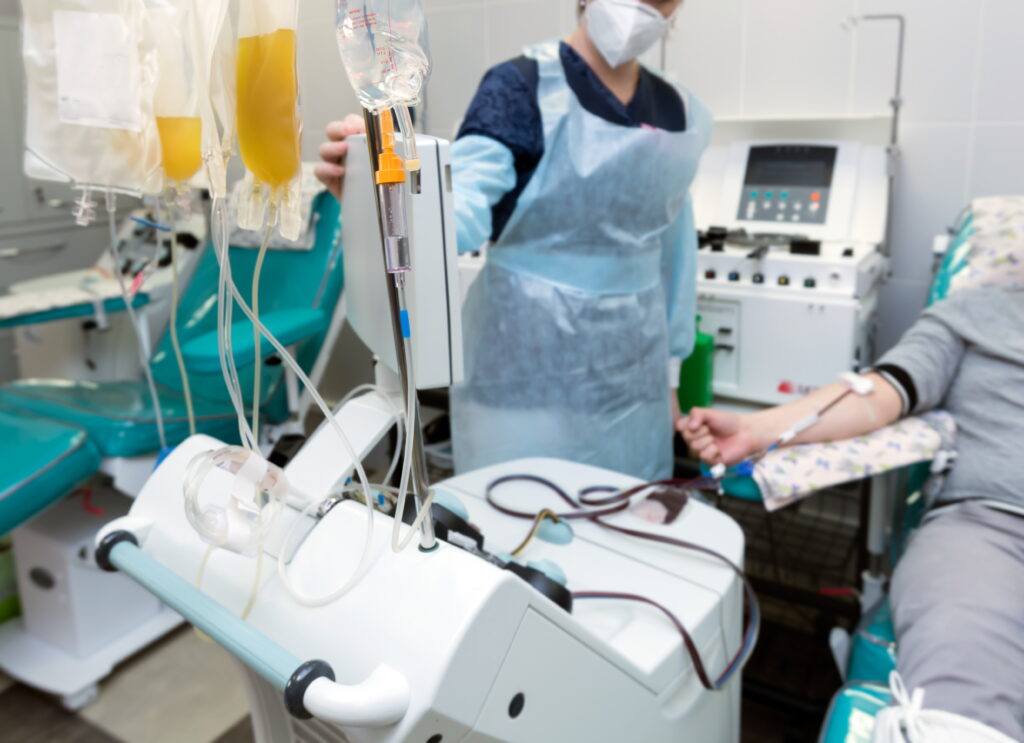
As immunotherapies continue to transform patient care, the biopreservation of apheresis-derived cells plays a vital role in ensuring consistency and quality. This blog explores how advanced preservation strategies—such as optimized cryopreservation and hypothermic storage—help reduce variability, protect cell function, and support the scalable manufacturing of cell-based therapies.
Navigating Current Standards and Compliance for Cell and Gene Therapy (CGT) Storage Containers

Adhering to stringent standards for Cell and Gene Therapy (CGT) storage containers is crucial to ensuring product safety and efficacy. From USP guidelines to regulatory requirements, this blog explores how proper materials, container closure integrity, and visual inspection processes safeguard the integrity of CGT products throughout their lifecycle.
CryoCase: Rethink the standard. Replace the bag.

Discover the groundbreaking CellSeal® CryoCase™, the first rigid primary container engineered for closed-system fluid processing in cell and gene therapy (CGT) applications. Launched in November 2024, the CryoCase offers unmatched fracture resistance, transparency for quality inspections, and automation-ready design, addressing the common pitfalls of traditional cryobags and cryovials. With proven performance in freeze-thaw processes and enhanced contamination control, CryoCase is revolutionizing CGT manufacturing workflows. Explore white papers, webinars, and case studies to see how CryoCase can transform your storage solutions. Learn more or request your test sample today!
BioLife Solutions Expands Evidence Library With Citations of Bioproduction Tools in Scientific and Clinical Research
BioLife Solutions’ refreshed Evidence Library offers a comprehensive collection of published data on the use of biopreservation tools in cell and gene therapy. With enhanced navigation and search capabilities, users can easily explore scientific evidence supporting the adoption of products like CryoStor®, HypoThermosol®, and more.
How the BioLife Solutions Master File Letter Request Process Works for CryoStor® and HypoThermosol® FRS

Cross-Referencing BioLife Master Files CryoStor and HypoThermosol FRS (HTS-FRS) are critical components in many customer cell and gene therapy (CGT) and regenerative medicine processes. Although they are not regulated medical products—meaning they are not classified as drugs, medical devices, or marketed/registered as excipients—they serve a critical role as ancillary/raw materials in cell-based manufacturing processes. BioLife […]
How Does a Water-Free Automated Thawing System Improve Your Cryopreservation Process?

Cryopreservation has come a long way… Over the past 50 years, advances in biopreservation techniques have enabled industry-wide progress in a range of fields including cell biology research, drug discovery, biobanking, assisted reproduction, and even plant and animal conservation, with the fields of cellular therapy and regenerative medicine seeing the greatest expansion recently. As these […]
Overcoming Cryopreservation Challenges

Navigating the Challenges of Cryopreservation in Cell-Based Research Biopreservation, and specifically cryopreservation, is critical for maintaining the viability of cells long-term throughout storage and research. Recently, BioLife Solutions partnered with Cell and Gene Therapy Insights to produce a poster that highlights many of the challenges researchers face, balanced with Biopreservation Best Practice suggestions. To receive […]
Journal Articles Citing CryoStor CS10® Recently Added to BioLife Solutions’ Evidence Library
Multiple Uses for CryoStor in Cell Manufacturing Journal articles recently added to BioLife Solutions’ Evidence Library illustrate several stages during the cell manufacturing process for which CryoStor CS10® cryopreservation solution is applicable. The three articles that follow include several different cell types cryopreserved in CryoStor CS10. An expanded list of cell types biopreserved in BioLife […]
Cellular Therapies for COVID-19 Highlighted During ISCT Paris 2020 (VIRTUAL)

The global impact of COVID-19 on our lives has been profound at virtually every level. Personally, professionally, politically, and economically, the pandemic impacts our lives, our relationships, and how we conduct business. We begin and end the day with the latest news on SARS-CoV-2, and up-to-the-minute COVID-19 data. In between, we must surmount any number […]
Journal Articles Citing Cryopreservation Component Recently Added to BioLife Solutions’ Evidence Library

Cryopreservation adds flexibility to timelines when working with cells. In the case of cellular therapies, that can mean initial flexibility to schedule patient cell collection, transport cells to the manufacturing facility and initiate manufacturing. Cryopreservation of the final cell therapy product adds flexibility to be able to schedule the return of cells to the patient […]