Insights

Meet the Makers: Innovations and Insights from The Cell Summit ’25

Designing the Ideal Cryostorage Container for CGT

The Cell Summit ’25: A Must-Attend Event for Innovators in Cell & Gene Therapy
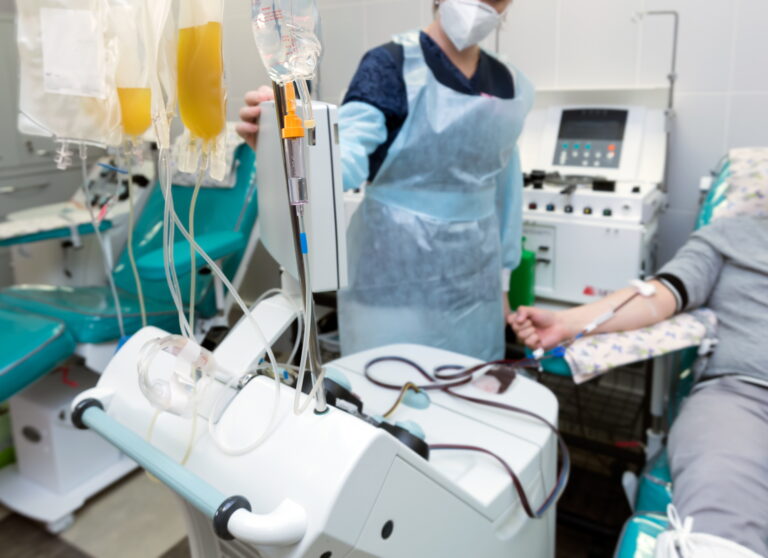
Optimizing Biopreservation Strategies for Apheresis-Derived Cells in Immunotherapeutic Applications

How to prepare early for regulatory submissions from IND to BLA

Navigating Current Standards and Compliance for Cell and Gene Therapy (CGT) Storage Containers

CryoCase: Rethink the standard. Replace the bag.
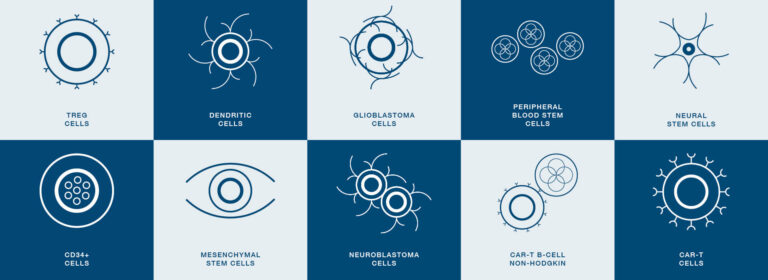
Customer Applications of CryoStor® and HypoThermosol® FRS in Cell & Gene Therapy and Regenerative Medicine
What COVID Taught Us About the Benefits of Cryopreservation for Cell-Based Therapies
BioLife Solutions Expands Evidence Library With Citations of Bioproduction Tools in Scientific and Clinical Research

How the BioLife Solutions Master File Letter Request Process Works for CryoStor® and HypoThermosol® FRS

How Does a Water-Free Automated Thawing System Improve Your Cryopreservation Process?
Overcoming Cryopreservation Challenges

The History of Cryopreservation

Universal Cell Management System – What is it? and Why is it important to the Cell and Gene Therapy (CGT) Industry?

Empowering Your Downstream Cell and Gene Therapy Processes: A Sexton Biotechnologies Focus
Loading more