cGMP Storage
Facilities
Biological and Pharmaceutical storage
We have GMP storage facilities located Nationwide, we work hard and pride ourselves in being able to service the entire country. We manage a broad range of products for our clients including ambient, controlled room temperature, refrigerated and frozen conditions. These projects can range from multiple pallets to a few cubic feet.
FDA regulations for component and active ingredients
Our GMP storage facilities enforce important FDA regulations for both component and active ingredients. This is especially important because many of our locations house pharmaceutical and biological samples. Some of the regulations we adhere to, and strictly enforce include:
- Premier inventory control and distribution processes
- Easy identification of biological material on the exterior of the samples
- Component testing of all samples that are distributed to customers
- Written instructions of how the sample is made
- All pharmaceutical and biological samples are placed within containers that provide complete closure. This helps eliminate the possibility of contamination.
The same customer service approach, which allows industry leading control and access to critical samples, is available whether storing one sample or 100 pallets. All of our New Jersey and Massachusetts GMP storage facilities integrate cold chain management and cGMP-controlled temperature storage. We can assure you that we will provide a reliable and comprehensive solution for the management of your critical pharmaceutical materials. Our team makes outsourcing of sample management affordable, easy, and more efficient for our clients.
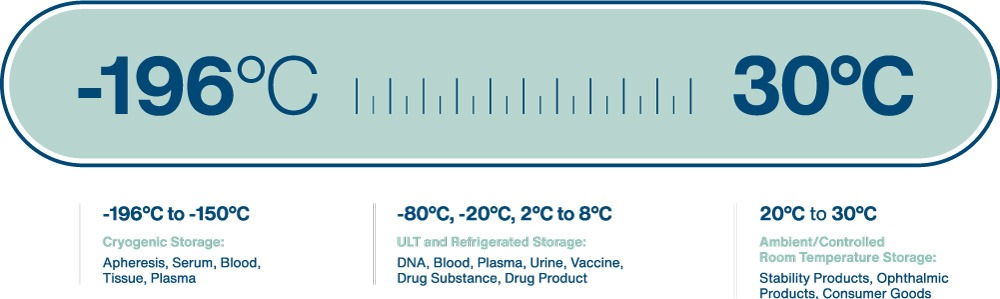
Sample types stored
Why work with SciSafe?
We work hard to maintain professional relationships with over 300 leading organizations throughout the country. We are currently located in New Jersey, Massachusetts, Utah and Amsterdam, but we service the entire world. The SciSafe team is always able to offer predictable and clear pricing and customizable solutions for our clients. As a cGMP storage company, we always follow standard operating procedures (SOPs) and remain dedicated to our bespoke industry.

SciSafe Centers of Excellence offer dedicated pharmaceutical and biological specimen storage in our state-of-the-art facilities.
Seven reasons to choose SciSafe:
SciSafe has built flourishing relationships with over 300 of the world’s leading and most admired organizations. Clients have repeatedly chosen to store their most valued and irreplaceable samples because they trust SciSafe to care for their samples as if they are their own. We value and respect our long-term relationships with our clients.
The SciSafe team consists of some of the leading voices in pharmaceutical and biological sample management. With over 60 years combined industry experience, SciSafe has one of the most experienced and knowledgeable teams in the field of commercial biorepositories.
Having established numerous cGMP biological and pharmaceutical storage facilities, the SciSafe team is uniquely qualified in all areas of regulatory compliance, cold chain distribution, storage protocols, equipment management, monitoring, and comprehensive sample management.
We don’t sleep so you can!
SciSafe’s industry leading customer service is designed to accommodate our clients’ specific needs. SciSafe operates 24/7/365 and with total dedication to the safety of our clients’ samples at all times. Continuous improvement of safety systems and contingencies is foundational to SciSafe’s core beliefs and its commitment to become the number one sample management company globally.
For over twelve years, SciSafe has managed millions of samples for hundreds of clients. This has been complimented by the best infrastructure and facilities available anywhere.
To ensure absolute satisfaction, SciSafe offers a unique CSMP (Customized Sample Management Plan) as part of our clients’ onboarding process. Our industry leading CSMP offers complete customization and flexibility to ensure that our clients’ sample management needs are clearly defined and fulfilled. No two customers are alike and SciSafe will not ask you to fit into the already established mold.
SciSafe builds long term and growing relationships with its clients. To do this, we offer clear predictable pricing based on the space that is utilized by the client. SciSafe does not charge for sample pulls, project set up, reports, or other sample management charges that are standard in the sample management industry. This allows clients to be able to budget correctly without fear of hidden or variable pricing. SciSafe offers same day delivery if located within a 50 mile radius of our facilities.
SciSafe has over 86 Standard Operating Procedures (SOPs) within its network of facilities. A robust Quality Plan and Corrective And Preventative Action (CAPA) system modeled after the FDA required systems (21CFR Parts 210 & 211) for pharmaceutical manufacturers make SciSafe a valued partner in every stage of drug development and distribution.
SciSafe is cGMP compliant. Its electronic systems including sample inventory, sample monitoring, chain of custody, document control and training are 21CFR Part 11 compliant. SciSafe attained CAP-BAP accreditation in 2023.
SciSafe facilities follow strict surveillance procedures with 24/7 video monitoring systems to protect samples. Purpose-built for secure sample storage, our facilities are auto-secured by card-controlled access to admit trained and certified staff, ONLY. All data backs up daily and direct messages to our facilities managers generates immediate response to security alerts. Equipment redundancy and backup generators are regularly maintained and load-tested to operate through automatic switches for guaranteed 24-hour biorepository operation.
Make
Contact

SciSafe Storage Services
Class-defining solutions for biological and pharmaceutical storage.